Latest News
18-Oct-2017Minilab Training in Mauretania
End of September, staff from the National Drug Quality Control Laboratory of Mauretania have been trained for one week in the operation procedures of the newly acquired Minilab developed by the Global Pharma Health Fund (GPHF). The trainer Nyaah Fidelis Bah Ngoh came from Cameroon where he works as a pharmacist in the Central Pharmacy of the Presbyterian Health Service.

The participants of the training in front of the National Drug Quality Control Laboratory in Mauretania. (Picture: Ngoh)
12-Oct-2017
Minilab workshop at the University of Tübingen
During the second course “Pharmacy in International Health and Disaster Operations” at the pharmaceutical institute of the University of Tübingen once again a special workshop on the GPHF-Minilab™ took place. Dr. Richard Jähnke, project manager of the GPHF, presented the mobile lab and instructed the participants in its proper use. For more information please see here.

Dr. Jähnke (centre) demonstrated the correct operation with the minilab. (Picture: Heide)
08-Sep-2017
International survey confirms the importance of the GPHF-Minilab™
Once again an international survey has confirmed the meaning of the GPHF-Minilab™ for the reliability and quality of medicine especially in low- and middle-income countries. By using the mobile lab of the GPHF in seven countries in Africa and Asia nearly 900 medicine samples were examined and 21 were confirmed to be substandard or falsified medical products. The survey has been run by the Difäm-EPN Minilab Survey Group, who is convinced, that surveillance for poor-quality medicines can be carried out by local organizations in low- and middle-income countries using a simple, low-cost technology. Such surveillance can identify an important subgroup of the circulating substandard and falsified medical products and can help to prevent them from causing harm in patients.
The survey, titled “Surveillance for falsified and substandard medicines in Africa and Asia by local organizations using the low-cost GPHF Minilab” now is published in PLOS ONE, a peer-reviewed open access scientific journal. For more information please see here.
24-Aug-2017
Minilab Workshop in Cameroon
In Limbe at Cameroon’s Atlantic coast once again a workshop on the use of the GPHF-Minilab™ was held. Participants of the one-week session were 14 representatives of the Ecumenical Pharmaceutical Network from Ghana, Malawi, Uganda, India and Cameroon. Trainer was Professor Lutz Heide from the Tübingen University in Germany. For more information please see here.
03-Jul-2017
GPHF started ten years ago
Exactly ten years ago, in July 2007, the Global Pharma Health Fund (GPHF) started his project work. At that time the GPHF succeeded the German Pharma Health Fund, a joint initiative of pharmaceutical companies in Germany to promote healthcare projects in developing countries. Since 2007 the GPHF is exclusively supported by Merck. The international science and technology company already was one of the founders of the German Pharma Health Fund back in 1985.
One of the main reasons for Merck to become the initiator of the new GPHF was to guarantee the continuation of the GPHF-Minilab™ project Back in 2007 the minilab had already proven its value to fight counterfeit drugs but with the backing of Merck the number of minilabs working all over the world increases rapidly. Today more than 820 labs in almost 100 countries are in place. Also the number of ingredients to be analyzed by the mobile lab rose from 40 to currently 90. Specialized minilab trainings, running in developing countries, became a regular part of the project too. Today, in many places the GPHF-Minilab is an important component of the local drug monitoring that helps to protect people from the deadly danger of counterfeit drugs.
08-Jun-2017
Once more the GPHF-Minilab™ identifies a fake medicine
Once again the GPHF-Minilab™ has unmasked a counterfeit medicine. By using the mobile lab, in the Democratic Republic of the Congo staff members of the Ecumenical Pharmaceutical Network detected a so called medicine to treat malaria completely lacking the active pharmaceutical ingredient. For more information please see here.

Counterfeit tablets with no quinine found in DR-Congo
04-May-2017
Minilab training in Cameroon
Like many African regions also the hospitals and healthcare facilities in northwest Cameroon near the Nigerian border are affected by the potential danger of counterfeited or substandard medicines. To test the quality of the drugs on site and to protect people from the danger of counterfeit drugs now for the first time a GPHF-Minilab™ should be used in the region. In a training course, which took place in the hospital in Njinikom, Dr. Richard Jähnke form the Global Pharma Health Fund (GPHF) instructed local healthcare staff in the use of the mobile lab. For more information please see here.

The St. Martin de Porres Catholic General Hospital in Njinikom hosted the training.
21-Mar-2017
Merck donates six GPHF-Minilabs to Sierra Leone
Merck now has made a donation of six GPHF-Minilabs, representing a total value of more than 30.000 Euro, for the use in Sierra Leone. The recipient of the mobile labs is the National Pharmaceutical Quality Control Laboratory in Freetown, the capital of Sierra Leone. The donation was initiated and financed by Merck Northwest Africa. The country organization has already supported the project work of the GPHF in the past.
Sierra Leone belongs to the poorest and least developed countries in Africa. The outbreak of the Ebola fever in 2014 has aggravated the humanitarian situation even further. The GPHF-Minilabs should contribute to a better quality control of the drugs provided on site to protect people from the danger of counterfeit and substandard drugs.
Merck, a leading science and technology company and at the same time the oldest pharmaceutical and chemical company in the world, supported the project work of the GPHF for a long time and is committed to the improvement of healthcare in Africa. The donation of minilabs is part of this engagement.
09-Mar-2017
Successful Project Work
During his general meeting the Global Pharma Health Fund drew a very successful balance on his project work in 2016. 64 GPHF-Minilabs were delivered, so in total, by the end of the year 795 mobile labs were situated in 95 countries worldwide. In addition to the execution of four special training courses on the Minilab in Kenya, Zambia, Rwanda and Mozambique the GPHF has again been able to integrate five new ingredients to the test methodology of the lab. Now 85 active ingredients can be analyzed. Also in the future, the focus of the project work of the GPHF will be on the further development of the Minilab, reconfirmed the participants of the meeting. At the general meeting Doris Meier was elected as the new treasurer of the Global Pharma Health Fund.
09-Feb-2017
GPHF-Minilab™ now capable of testing 85 drug compounds
The Global Pharma Health Fund (GPHF) is pleased to announce the release of more test protocols for its Minilab, a self-contained mini-laboratory for rapid drug quality verification and easy detection of falsified medicines. The Minilab is particularly suitable for areas of the world where counterfeiting is prevalent but sophisticated tools for their detection are not readily available.
Minilab users clearly benefit from this performance improvement, for example the identification and content verification of more vital medicines to treat infectious and cardiovascular diseases (cefpodoxime, chlorhexidine, dapsone, efavirenz and amlodipine). The new method inventory includes also prevailing fixed-dose combination formulations for amlodipine and efavirenz, for example amlodipine combined with HCT or atenolol and efavirenz combined with tenofovir, emtricitabine or lamivudine. The gap on essential antiretrovirals is closed with an update on current nevirapine FDCs.
The latest Minilab supplement is published jointly with the Promoting the Quality of Medicines (PQM) programme run by the United States Pharmacopeial Convention (USP). Demo versions of the new test methods can be accessed in English, French and Spanish on this homepage. Printed copies are available at our logistic partner Technology Transfer Marburg (TTM).
24-Jan-2017
GPHF-Minilab™: Training course in Zanzibar
In January, sixteen people from medicines inspectorates and food and drugs boards from Tanzania, Kenya, Uganda, Burundi and Rwanda have been trained on the use of the GPHF-Minilab™, the mobile test kit of the Global Pharma Health Fund (GPHF) with simple test methods for rapid drug quality verification and counterfeit medicines detection. Tanzania, Kenya, Uganda, Burundi and Rwanda are all part of the East African Community (EAC), and – like many other regions in the world – the EAC is affected by circulating counterfeit medicines. The one week training session, financially supported by the German Ministry of Economic Cooperation and Development, took place in Zanzibar, a semi-autonomous part of Tanzania, and was held by Dr. Richard Jähnke from the GPHF. To fight counterfeit drugs in the EAC, a trainings session on the GPHF-Minilab has already been handled in Rwanda last year and third session will take place in Burundi. For more information please see here.

The participants of the training session in Zanzibar.
01-Dec-2016
Minilab Training Sessions in Mozambique and Cameroon
In November two training sessions on the GPHF-Minilab™ has been held in Mozambique and in Cameroon. While the training in Mozambique was led by Dr. Richard Jähnke from the Global Pharma Health Fund (GPHF), the course in Cameroon was run by Tambo Cletus, a pharmacist from the health department of the Cameroon Baptist Convention. In Mozambique and in Cameroon too, participants of the training sessions were staff members of local health facilities who use the mobile lab of the GPHF for the drug quality control in their countries. For more information please see here.

Dr. Richard Jähnke (left) led the training session on the minilab in Mozambique.
16-Nov-2016
Best Practice
The Access to Medicines Index 2016, published on November 14th, ranks the GPHF-MinilabTM as one of the exemplary international healthcare projects. The Access to Medicine Index is an independent initiative that ranks the world’s 20 largest research-based pharmaceutical companies according to their efforts to improve access to medicine in low- to middle-income countries. Among the leading companies only one German company is represented: Merck. Through its fundings Merck enables the projects of the GPHF for a long time. Meanwhile more than 750 mobile GPHF-Minilabs to detect counterfeit drugs are in operation all over the world. Further projects by Merck rated in the index are the donation of medicines to fight bilharziosis and a national program to control malaria in Namibia. The Access to Medicine Index is published every two years by the Access to Medicine Foundation, an international not-for-profit organisation dedicated to improving access to medicine for people in need. The organisation receives financial support from donors such as the Bill & Melinda Gates Foundation and the Dutch Ministry of Foreign Affairs. For more information please see http://accesstomedicineindex.org.
19-Oct-2016
Merck donates Minilabs to the Ivory Coast
Last week, Merck Germany, a leading science and technology company, donated two Minilabs to the Ivory Coast (West Africa) thus indicating its support in fighting counterfeit medicines. The handing over of the Minilabs took place during the opening of Merck’s new office in the capital of Abidjan. The Ivory Coast’s First Lady, Dominique Claudine Ouattara and the Minister of Health and Public Hygiene, His Excellence Doctor Raymonde Goudou Coffie attended the office opening and Minilab donation ceremony.
The Minilab is a mobile mini-laboratory that helps in the detection of counterfeit medicines with simple but reliable test methods for rapid drug quality verification. Almost 800 Minilabs have been supplied to health facilities worldwide so far. Due to the donation of Merck, the Minilab is now also available in Côte d’Ivoire. The Minilab development and project work is run by the Global Pharma Health (GPHF); Merck’s charity arm in Frankfurt (Germany).

Frank Gotthardt (left), Vice Chairman of the GPHF, presented the donation.
29-Aug-2016
Once again the GPHF-Minilab™ unmasks a fake drug
Thanks to the GPHF-Minilab once again a fake drug was identified. In Cameroon, staff members of the Ecumenical Pharmaceutical Network (EPN) discovered a so-called antimalarial medicine without any active ingredient. Investigations have also shown that the named manufacturer does not exist. Recently the EPN has already ensured fake antimalarial medicines in the DR-Congo. Meanwhile the World Health Organization has published an official Medical Product Alert. For more information please see here.

Fake antimalarial medicines with zero quinine content found in Cameroon.
09-Aug-2016
Plea for the use of the GPHF-Minilab™
For the first time at Tübingen University’s Institute of Pharmacy the course “Pharmacy in Development Cooperation and Emergency Aid” has begun. The course is intended for pharmacy students and registered pharmacists. Professor Lutz Heide (Tübingen) noted in this context, that counterfeit drugs are serious problem in many development countries. He pleaded for the use of analytical methods by which the quality of drugs could be verified also under difficult conditions. According to Professor Heide, the Minilab of the Global Pharma Health Fund, which is also used during the course, is ideally suited for this purpose.

At the University Tübingen the participants of the course “Pharmacy in Development Cooperation and Emergency Aid” learn how to handle the GPHF-Minilab™ too. (Picture: University Tübingen)
16-Jun-2016
Minilab training in the East African Community
For the first time, the Global Pharma Health Fund (GPHF) conducted a training course on the GPHF-MinilabTM for the member states of the East African Community (EAC). Onset this month, in Kigali, the capital of Rwanda, GPHF representative Dr. Richard Jähnke trained staff from medicines regulatory and inspection agencies from five EAC countries. The GPHF-Minilab, a mobile lab to identify counterfeit drugs, was developed by the GPHF and is already used in more than 90 countries worldwide. Around 170 million people live in the member states of the EAC, Kenya, Uganda, Tanzania, Burundi, Rwanda and South Sudan. The cooperation in the health care sector and the harmonization of related regulations facilitating access to quality-assured medicines is one of the tasks of the EAC. In this context, an important role will be played by the GPHF-Minilab, too. For more information please see here.

Richard Jähnke (PhD) and the participants of the Minilab training course in Kigali.
23-May-2016
Anniversary: GPHF-Minilab™ No. 750 will be delivered
The Global Pharma Health Fund e. V. (GPHF) is proud to announce that its 750th minilab for the detection of counterfeit drugs will be delivered in the next days. The distribution partner of the GPHF, Technologie Transfer Marburg (TTM), delivers the mobile lab first to Belgium, but the final destination of the lab will be the East African Rwanda. In Rwanda the minilab becomes part of a comprehensive project for the education and training of health technicians in the East African Community (EAC). This project is largely supported by the German Federal Ministry for Economic Cooperation and Development (BMZ) in the framework of a new specific initiative for Africa. In this context the Federal Institute of Physical Technology in Braunschweig (PTB) – as implementing organisation - has already ordered eight minilabs for the future use in Rwanda. The place of use of all the minilabs will be a new training center at the University of Rwanda in Kigali where training courses for the member states of the EAC are being planned. Dr. Richard Jähnke from the GPHF will travel to Rwanda soon for an initial central training session on the use of the GPHF-Minilab.
04-May-2016
First Minilab training mission in Zambia
For the first time, the GPHF carried out a training session on its GPHF-MinilabTM in Zambia. Teachers from the University of Zambia in Lusaka and staff from medicines regulatory and drug inspection have been trained in the use of the mobile laboratory by Dr. Richard Jähnke from the GPHF for one week end of last month. In the future the GPHF-Minilab should become part of the professional training of pharmacy students and pharmaceutical technicians at the University. As many countries in Africa, Zambia is affected by counterfeit drugs, too. Due to the integration of the Minilab into university education on industry pharmacy the responsible authorities hope for an improvement of the current situation. The establishment of an industry pharmacy course at the University of Zambia is supported by several multilateral medicines supply chain and quality assurance programmes maintained by the U.S. Agency for International development. For more information please see here.

The end of a successful week: Minilab training in Zambia.
22-Feb-2016
Tanzania: Assistant pharmacists will be trained on the GPHF-Minilab™
Through the donation of four minilabs and accompanying training sessions last year the GPHF supported a project on the education of assistant pharmacists in Tanzania. The training on the GPHF-Minilab™ to detect counterfeit drugs now has become part of the official curriculum for assistant pharmacists in Tanzania too. The engagement of the GPHF in Tanzania is part of a larger project aiming the training of assistant pharmacists which is run by the German Society for International Cooperation (GIZ), the companies Merck, Boehringer Ingelheim and Bayer and various clerical organizations.
15-Feb-2016
GPHF-Minilab™ now capable of testing 80 drug compounds
Once again the GPHF has extended the applications of his minilab to detect counterfeit drugs. The mobile unit now is capable of testing 80 drug compounds. The method extension includes five more antibiotics. The GPHF-Minilab, already used in more than 90 countries, offers a quick, reliable and cost-effective way to detect counterfeit drugs. For more information please see here.
03-Feb-2016
Minilab Training in Kenya
GPHF Project Manager Dr. Jähnke carried out a training session on the GPHF-Minilab™ in Kenya last week. Within the scope of an international program to promote the quality of medicine 28 pharmacists have been trained in the use of the minilab. Kenya’s drug regulatory authority expressed its interest in institutionalizing the use of Minilabs in many regions of the country. For more information please see here.

Dr. Jähnke explaining the use of the GPHF-Minilab™.
28-Jan-2016
Minilab Detects Falsified Tablets Circulating in Cameroon
During a recent medicines quality survey in Cameroon, a batch of falsified Clomid 50 mg tablets missing more than 90% of the active pharmaceutical ingredient have been detected using Minilabs of the Global Pharma Health Fund (GPHF) designed for rapid medicine quality verification. For more information please see here.
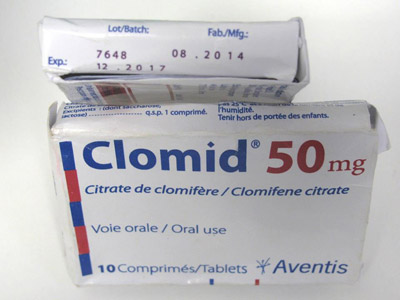
Spurious “Clomid 50 mg” batch missing 90% of its active pharmaceutical ingredient clomifene citrate. (Picture: DIFAEM.de)
30-Oct-2015
GPHF-Minilab detect counterfeit antibiotics
With the GPHF-MinilabTM users from the Ecumenical Pharnaceutical Netweork (EPN) now detect counterfeit antibiotics in the Democratic Republic of Congo in Central Africa. The counterfeit medicines contain no active pharmaceutical ingredient and represent a serious health risk. For more information please see here.

Counterfeit antibiotics detected by the GPHF-Minilab in Congo. (Picture: DIFÄM)
13-Oct-2015
Minilab Capacity Building in Angola
Twenty-two staff from the Angolan medicine regulatory authority, drug inspectorate, central medical store and the national malaria programme completed a training on the use of the Minilab basic tests and the operational framework for implementing a nationwide drug quality monitoring programme in Luanda, the capital of Angola, last week. For more information please see here.

Minilab training course in Lunada, Angola.
