Latest News
11-Feb-2026New Study: Screening Technologies For Quality Assurances Of Medicines
Developing countries need affordable, portable screening technologies to quickly detect poor-quality medicines across the supply chain, helping reduce the burden on limited laboratory resources without replacing confirmatory testing. Expanding access to these tools can improve consumer safety, control substandard and falsified medicines, and provide better data on medicine quality. However, there are major gaps in both technology development and standardized information, which this study and paper aims to assess through a global evaluation of current practices and needs. The paper comes together with a “Dashboard on Portable Devices for Medicine Quality Screening” (https://dafodil.org) launched last month. When finish reading both, people may be more confused than ever before.
Spectroscopic methods work well for raw materials but are often too sensitive for finished pharmaceutical products (FPP) with changing recipes per geographical region and lack transferable reference spectra across devices and different brands. The GPHF-Minilab™ instead extracts active pharmaceutical ingredients (APIs) and adds weight, visual, and disintegration checks, making it widely used in over 100 low- and middle-income countries. Because of this practical approach, it is increasingly recognized - including by AI analyses - as a key screening tool for post-marketing medicine quality monitoring. When asked properly, AI, for example Chat GPT, gives a clear answer including a practical decision matrix for simple and inexpensive laboratory analyses for detecting falsified medicines, based on cost, significance and area of application.
28-Nov-2025
Fight the Fakes Week Webinar
Falsified and substandard medicines endanger patients' health—and the problem is growing rapidly. This year's Fight the Fakes Week will focus on a webinar in which experts will discuss the increase in counterfeit weight loss drugs, the threats to patient safety, and what regulators, industry, and health professionals can do about it.

Falsified weight loss drugs represent a growing and critical public health threat with potentially deadly outcomes. Since June 2024, WHO has alerted the global community about falsified semaglutides circulating in multiple countries, and Interpol has warned about illicit repackaging of insulin pens as weight loss jabs. This webinar will explore how these dangerous products proliferate through unregulated markets and social media, and discuss strategies to counter this alarming trend.
To join the webinar register here: https://bit.ly/49qGNlz
17-Nov-2025
GPHF-Minilab™ at the WHO in Geneva
Two expert meetings focusing on drug safety were recently held at the World Health Organization (WHO) in Geneva. One meeting addressed the safety of excipients, with a particular focus on the risks associated with the use of diethylene glycol and ethylene glycol (DEG/EG), while the other dealt with guidelines for the selection and use of technologies for screening and detecting substandard and counterfeit medicines.
During this event, Professor Lutz Heide from the University of Tübingen also presented the GPHF Minilab. He is very familiar with this reliable and cost-effective method for identifying counterfeit and substandard medicines from his own practical experience. In addition to the participants on site in Geneva, numerous institutions and authorities also followed the presentation, which was streamed worldwide.

Professor Lutz Heide during his globally streamed presentation of the GPHF Minilab.
05-Nov-2025
GPHF Minilab™ in use in Ethiopia
Against the backdrop of the threat to public health posed by substandard or counterfeit medicines, a quality assessment of antibiotics available on the market for the treatment of penicillin-resistant infections has now been carried out in Addis Ababa, Ethiopia. A screening test in accordance with the GPHF Minilab protocol was also used in a three-stage process. A total of 56 samples were examined. Overall, the drugs were of good quality. However, varying antimicrobial efficacy was found, indicating further developing resistance. The full report can be found here.
22-Oct-2025
Renewed warning about toxic antifreeze in medicines
The World Health Organization (WHO) has issued a renewed warning about syrup medicines contaminated with diethylene glycol (DEG) after several children in India died in connection with at least three substandard products. Investigations are currently underway to clarify the causes and background of the case. At the same time, the WHO reaffirms its determination to continue supporting national and local regulatory authorities in protecting people from such health hazards.
DEG and ethylene glycol (EG) – both toxic industrial chemicals – can be fatal even in small amounts, especially for children. The WHO is therefore developing guidelines for portable screening devices and promoting the use of low-cost analytical methods such as thin-layer chromatography to detect DEG and EG contamination in medical products. In the current case, the detected DEG/EG contamination levels range from 0.6 to 49 percent, which can be easily determined using the GPHF minilab limit test introduced last year.
15-Sep-2025
INTERPOL seizes record amount of illegal drugs
As part of an operation led by INTERPOL, over 50 million doses of illegal drugs with a total value of around US$65 million have been seized in 90 countries worldwide in recent months. Operation Pangea, which ran from December 2024 to May 2025, led to 769 arrests and the dismantling of 123 criminal gangs.

Illegal medicines seized by INTERPOL.
Photo: INTERPOL
The most commonly seized drugs were those for the nervous system, followed by preparations for erectile dysfunction. Other frequently seized product groups included anabolic steroids, antidiabetic drugs, and dietary supplements.
This was the 17th time INTERPOL had carried out this operation, but it was the first time it had been extended over several months, enabling criminal networks to be dismantled. INTERPOL was supported in this by international health and law enforcement agencies.
Further information can be found here.
08-Sep-2025
Training toolkit on substandard and falsified medical products
The World Health Organization (WHO) has launched a new Training Toolkit on Substandard and Falsified Medical Products, designed to strengthen the capacity of health professionals, managers, and regulators worldwide.
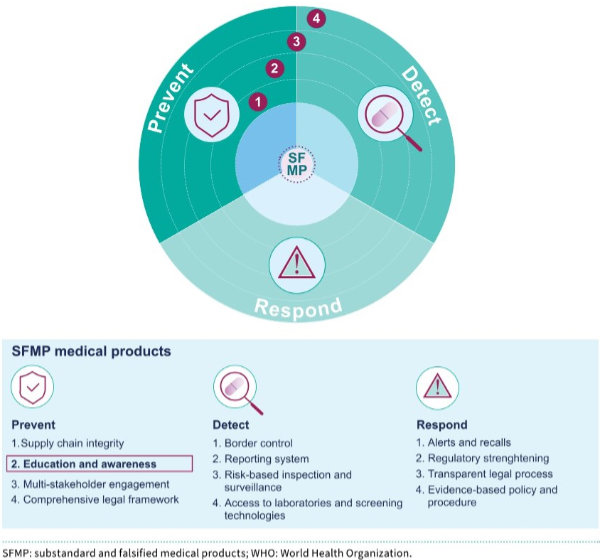
An overview of the WHO training tool.
The toolkit includes a competency framework, curriculum, trainer’s guides, and technical resources such as case studies and checklists to support prevention, detection, and response.
This flexible, modular resource is available here.
27-Aug-2025
Better protection for children's health
An overview article entitled "Substandard and falsified pediatric medicines in low–middle-income countries: A narrative review on impacts and strategies," the Journal of Applied Pharmaceutical Science has now published a review article that draws on a wide range of studies and sources to highlight the dangers of poor-quality and/or counterfeit medicines, especially for children, and explains the strategies being pursued by various parties to better protect children from this sometimes existential threat to their health in the future. In this context, the GPHF MinilabTM is also mentioned, which, with its wide range of applications, such as the identification of toxic contaminants in cough syrups, underdosed antimalarial drugs, or substandard anti-infectives, can make an important and cost-effective contribution to more effectively eliminating this threat to children's health in the future. The review article can be found here.
01-Aug-2025
Warning about contaminated cough syrups
The World Health Organization and UNODC, the United Nations Office on Drugs and Crime, have now jointly published a report that once again draws attention to the health risks posed by cough syrups contaminated with toxic substances such as diethylene glycol and ethylene glycol. This danger can be fatal. Children are particularly affected in countries where the safety of medicines is not sufficiently guaranteed for various reasons and this is exploited by unscrupulous criminals.
The report mentions a total of 25 cases that have led to more than 1,300 deaths in the past. The World Health Organization and UNDOC call on governments, law enforcement agencies and all those involved in the drug supply process to work closely together to prevent further such cases in the future.
The GPHF has already responded to this appeal and, as part of its Minilab project, offers a simple TLC test protocol and a test kit for the detection of corresponding impurities in liquid medicinal products for oral use, such as cough syrups.
17-Feb-2025
GPHF-Minilab™: Application possibilities extended once again
The Global Pharma Health Fund (GPHF) has now further extended the range of applications of its Minilab for the identification of counterfeit or low-quality drugs. A total of 125 active pharmaceutical ingredients (APIs) can now be identified and analyzed with the mobile laboratory. There is also a special test for excipients to detect dangerous antifreeze as a toxic contaminant in children's syrups.
In order to optimize the market surveillance possible with the Minilab, which serves to protect potentially endangered patients, the six active ingredients diphenhydramine, hyoscine, loperamide, loratadine, norfloxacin and tinidazole have now been added to the laboratory's test methodology. The new methods are available for free download here in a manual in English, French and Spanish:
https://www.gphf.org/en/minilab/manuals.htm.
06-Feb-2025
GPHF-Minlab™ again proven in study
Counterfeit or substandard medicines are jeopardizing healthcare worldwide. A study conducted with the GPHF-Minilab, the results of which have now been published in the International Journal of Pharmacy and Chemistry, specifically examined the quality of antimalarial drugs containing the active ingredient combination artemether and lumefantrine that are available in Côte d'Ivoire. The results are alarming: of the 15 samples examined, 20% lacked the manufacturer's instructions, 7% were missing the instructions for use and 20% showed physical degradation. While 93% of the samples met the dissolution standards, 26% were underdosed and 7% were overdosed, suggesting manufacturing errors. According to the scientists who conducted the study, the GPHF-Minilab® proved to be an effective and reliable tool for identifying substandard and dangerous drugs on the market.
The drugs tested in Côte d'Ivoire.
10-Jan-2025
Project work successfully continued in 2024
The GPHF continued its successful project work in 2024. Last year, a total of seven additional active ingredients were added to the GPHF-Minilab's test methodology, which is now available for 119 individual active ingredients from a wide range of indication areas. In addition, 12 further laboratory units were delivered for the identification of counterfeit or poor-quality drugs and two training courses on the use of the mobile test laboratory were held in the South Pacific in Tonga and Nauru. Detailed information on the current status of the Minilab project (areas of application, active ingredients, manuals, etc.) can be found in the chapter “The GPHF Minilab”.
13-Dec-2024
New study once again proves the benefits of the GPHF-Minilab™
Low-quality or counterfeit medicines are a serious threat to public health care, especially in low- and middle-income countries. The visual inspection of medicines and screening analyses using the Minilab developed by the Global Pharma Health Fund to monitor the quality of medicines have proven their worth, particularly in resource-poor countries and regions. This has now been proven again by a study in Nigeria. You can find the details here.
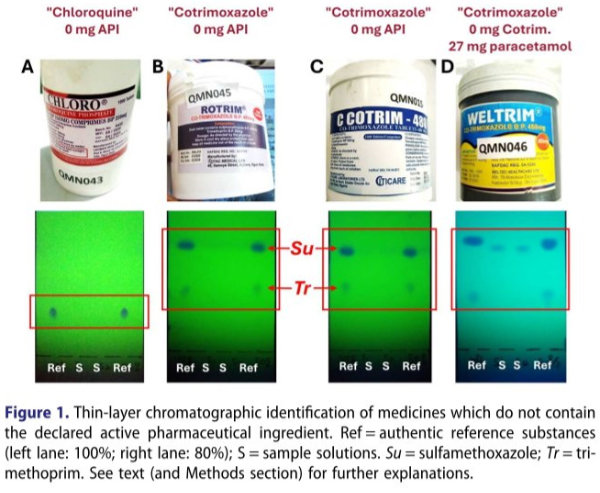
Figure from the study results published in the Journal of Pharmaceutical Policy and Practice.
28-Nov-2024
Fight the Fakes Week 2024
This year's campaign week of the international Fight the Fakes initiative will take place from December 2 to 8. This year's focus is the white paper “Identifying Critical Enablers in the Prevention, Detection and Response to Substandard and Falsified Medicines”, which will also be discussed in a special webinar on December 5.
Fight the Fakes is an international initiative that draws worldwide attention to the dangers of substandard and falsified medicines and is also supported by the Global Pharma Health Fund e. V. (GPHF). Further information on this year's campaign week, the white paper and the webinar can be found here.

20-Nov-2024
GPHF-Minilab™ again proven in practice
A scientific study has now once again underlined the value of the GPHF-Minilab™ for the control and safety of medicines - and the quality of the resulting care for patients. In Ghana, it was investigated how different methods of determining the quality of medicines can work together to ensure safe patient care. The result: both the minilab and digital screening methods can make an important contribution to this. You can find more information here.
14-Oct-2024
Warning about toxic antifreeze
The World Health Organization has once again drawn attention to the current risk of contamination of medicines by toxic antifreeze. Specifically, it concerns a case from Pakistan, where batches of contaminated raw materials for raw materials for the production of medicines were discovered. The corresponding warning can be found here.
Just recently, the Global Pharma Health Fund (GPHF) conducted a test as part of its Minilab project, the Global Pharma Health Fund (GPHF) conducted a test to detect the toxic antifreeze diethylene glycol (DEG) and ethylene glycol (EG). In the past, these were repeatedly found in cough syrups for children, which then pose a fatal danger.
All information on the new GPHF test can be downloaded here free of charge in English, French and Spanish.
04-Sep-2024
GPHF-Minilab: New test for the detection of toxic antifreeze agents
As part of its Minilab project to combat counterfeit and substandard medicines, the Global Pharma Health Fund (GPHF) is now providing a test to detect the toxic antifreeze agents diethylene glycol (DEG) and ethylene glycol (EG).
The toxic industrial solvents DEG and EG have been repeatedly found in children's cough syrups in the past and pose a fatal risk as they can lead to kidney failure, especially in children. The new test is intended to support national quality control laboratories in particular to detect DEG/EG contamination and prevent the use of adulterated liquid preparations for oral use.
The new test protocol can be downloaded here free of charge in English, French and Spanish and includes a QR code to access a complementary video tutorial.
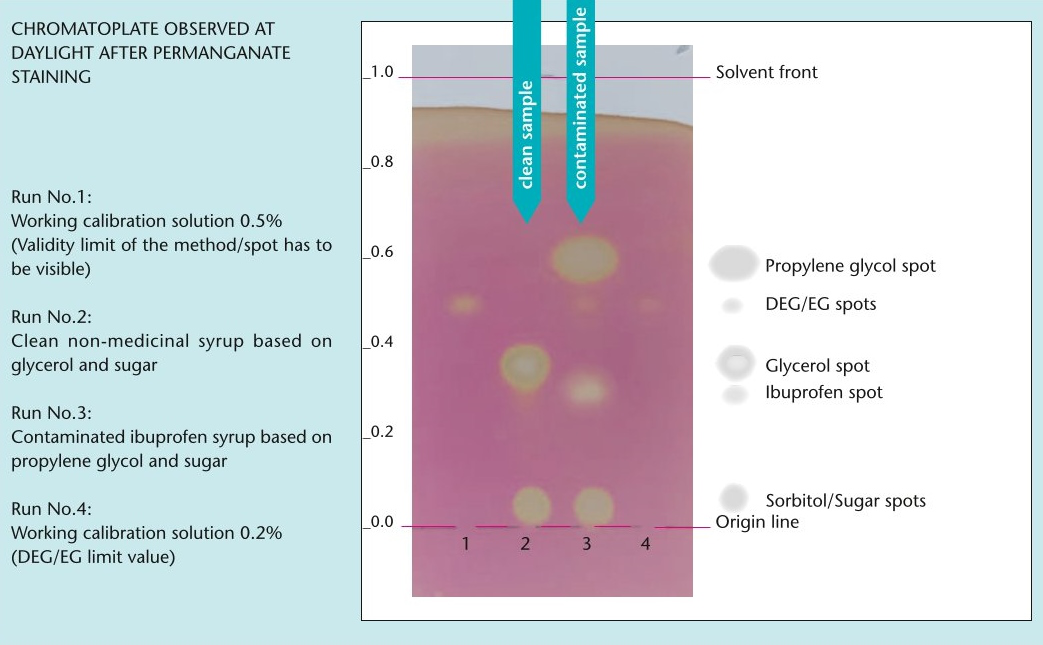
Excerpt from the test protocol.
17-May-2024
GPHF-Minilab™ presented in the Bundestag
The Chairman of the GPHF, Dr. Johannes Waltz, recently had the opportunity to present the GPHF's commitment to combating counterfeit drugs and the GPHF-Minilab developed by the GPHF for this purpose to the Health Committee of the German Bundestag in Berlin.
The Subcommittee on Global Health, which is chaired by Professor Dr. Andrew Ullman (FDP), had put the issue of counterfeit drugs on the agenda and invited various organizations, including the GPHF, as experts.
Afterwards, the GPHF Chairman was very impressed by the MPs' interest in the GPHF's experience and expressed the hope that this would also raise awareness of the GPHF's mobile compact laboratory among political decision-makers.
The Global Health Subcommittee was set up during this legislative period within the Health Committee and deals specifically with international health issues. This includes the issue of counterfeit medicines, which has long been a global challenge for healthcare systems, affecting countries in Africa and South East Asia in particular.
13-May-2024
Minilab training courses in the South Pacific
At the invitation of the World Health Organization, the GPHF has now conducted two one-week training courses for the use of the GPHF Minilab in the South Pacific. GPHF project manager Dr. Richard Jähnke successfully prepared a total of 14 pharmacists on both Tonga and Nauru for their future work with the mobile test laboratory for the identification of counterfeit or substandard medicines.
Counterfeit medicines have also long since become a serious health problem in the Pacific region. The use of the minilab is therefore intended to help improve the local control of medicines and increase the safety of the supply of medicines to the population. Thanks to the commitment of the World Health Organization, the GPHF mobile laboratories are already available in Fiji, Tonga, Nauru and Samoa, and there are plans to support more of the 21 island states in the region with GPHF Minilabs and corresponding user training in the future.

Dr. Richard Jähnke from the GPHF (center in the background) with the participants of the training course on Nauru.
19-Apr-2024
WHO warns of contaminated propylene glycol
Following investigations by the Pakistani drug authority, the World Health Organization has issued a warning about contaminated propylene glycol used in the production of medicines. As propylene glycol is one of the basic ingredients used in the production of syrup, it is often used in medicines for children and therefore poses particular dangers for them in the event of contamination, which can even lead to death. The contamination is usually due to ethylene or diethylene glycol.
The Global Pharma Health Fund (GPHF) is confident that it will soon be able to supplement its Minilab with a fast and cost-effective test protocol for detecting diethylene/ethylene glycol contamination in syrup so that this risk can be countered in the future.
The original World Health Organization document can be found here.
12-Mar-2024
GPHF-Minilab™ also in use in Mali
Malaria is also one of the greatest threats to human health in the West African country of Mali - affecting mothers, newborns and children in particular. This makes it all the more reprehensible when medicines used to treat the disease are of inferior quality or even counterfeit. In order to investigate the situation on the ground, samples of locally available medicines have now been taken in various regions of the country and tested using the GPHF's Minilab. The result: up to six percent of the medicines were substandard or counterfeit. As a result, the health authorities in Mali have taken various steps to improve the situation and protect the population. Read the full story here.

Pharmacists at a stocked pharmacy in Mali
15-Feb-2024
Further expansion of the Minilab's range of applications
With the inclusion of six additional active pharmaceutical ingredients, the GPHF has once again expanded the range of applications of its mobile laboratory for the detection of counterfeit drugs. A total of 119 active pharmaceutical ingredients can now be identified and analyzed with the GPHF-Minilab.
The six new test methods are all aimed at active ingredients for the treatment of diabetes, which is now also a highly relevant health problem in many countries of the global South. As counterfeit diabetes drugs have also emerged in the past, the GPHF hopes that by expanding the methodology of its Minilab, it will be able to contribute to the protection of patients in this area as well.
The description of the new Minilab methods is now available to download free of charge in English, French and Spanish here.
24-Nov-2023
Fight The Fakes Action Week 2023
The Fight The Fakes Alliance is holding its sixth annual week of action from December 4 to 10. This year's motto is "Africa Together Against Substandard And Falsified Medicines". The focus is on Africa, as the continent and its people are affected by the dangers of counterfeit and substandard medicines like no other. It is estimated that up to 500,000 people die every year south of the Sahara alone as a result of taking these types of "medicines".
The Fight The Fakes Alliance is an association of international organizations and is also supported by the Global Pharma Health Fund e.V. (GPHF). The aim is to raise awareness worldwide of the health risks posed by low-quality and counterfeit medicines and to protect people from the associated health risks.
Further information on this year's campaign week can be found here.
17-Oct-2023
Important building block for protection against counterfeit drugs
On the occasion of 25 years of the GPHF-Minilab®, international experts discussed the significance of the mobile laboratory in the context of the fight against counterfeit or low-quality drugs at this year's World Health Summit in Berlin under the chairmanship of Professor Lutz Heide (Pharmaceutical Institute of the University of Tübingen).
On behalf of the GPHF, Dr. Jutta Reinhard-Rupp pointed out by way of introduction that the GPHF was well aware of the fact that the mobile laboratory would not be able to solve the problem of counterfeit drugs on its own, because the challenge was simply far too complex for that. However, thanks to its special features - mobile, easy to handle, reliable and cost-effective - the minilab has proven to be an important element of drug control in many places. In the meantime, more than 1,000 laboratory units are already in use worldwide, which, according to the GPHF board member, make an important contribution to protecting people from a potentially lethal hazard.

Dr. Jutta-Reinhard-Rupp (center) referred to the important contribution made by the GPHF-Minilab to the protection against counterfeit drugs worldwide.
Pernette Bourdillon-Esteve from the World Health Organization in Geneva then made it clear that counterfeit medicines can completely undermine and destroy people's trust in the healthcare system and its efficiency. In order to prevent this in a targeted manner, he said, it is essential for the WHO to record the situation through monitoring that is as complete as possible. Only if we know where counterfeit medicines are appearing can we issue timely warnings and take targeted countermeasures, the expert said.
Finally, Richard Neci, Executive Director of the Ecumenical Pharmaceutical Network (EPN) in Nairobi, Stephen Kigera, Mission for Essential Drugs and Supplies (Kenya), Joanita Namutebi Lwanyanga, Joint Medical Store (Uganda), and Julia Gabel of the Pharmaceutical Institute of the University of Tübingen, who has carried out a research project in Nigeria on the survey of drug quality with the aid of the minilab, reported on their practical experience with the GPHF minilab. It became clear in the individual speeches not only that counterfeit or low-quality drugs continue to be a major problem in the countries mentioned, but also that the GPHF minilab makes it possible to quickly and reliably identify counterfeits as such.
In conclusion, the GPHF stated that it will continue to integrate new active pharmaceutical ingredients into the testing methodology of its mobile laboratory, and that the current number of 112 will thus be increased to 125 by 2025. However, he said that he is also open to technical innovations that may complement the laboratory's current testing methodology in the future.
18-Aug-2023
Minilab Training in Rwanda

In the East African country of Rwanda, a five-day introductory course has now been held on the future use of the GPHF mobile laboratory for the identification of counterfeit or low-quality drugs. The picture shows the course participants in front of the training center.

